skip to main |
skip to sidebar
Atoms
Atom is the smallest particle of an atom!
Atom
I INTRODUCTION

Evolution of the Model of the Atom
As scientists learned about the structure of the atom through experiments, they modified their models of the atom to fit their data. British physicist Joseph John Thomson understood that atoms contain positive and negative charges, while British physicist Ernest Rutherford discovered that an atom's positive charge is concentrated in a nucleus. Danish physicist Niels Bohr proposed that electrons orbit only at set distances from the nucleus, and Austrian physicist Erwin Schrödinger discovered that electrons in an atom actually behave more like waves than like particles.
Atom is the smallest unit of a chemical element that can exist. In ancient Greek philosophy the word “atom” was used to describe the smallest bit of matter that could be conceived of. This “fundamental particle”, to use the present-day term for this concept, was thought of as indestructible; in fact, the Greek word for atom (atomos) means “not divisible”. Knowledge about the size and nature of the atom did not begin to be acquired until long after the beginnings of experimental science in the 16th and 17th centuries. Although many of the new “experimental philosophers” believed in the reality of atoms, the progress of science owed little to the idea. The first quantitative explanation of the behaviour of matter in terms of atoms was attempted by Daniel Bernoulli in 1738, but his work was largely ignored. However, chemistry was discovering things about matter that only the idea of atoms could explain. Chemists recognized that all liquids, gases, and solids can be broken down into their ultimate components, or elements. For example, salt is a chemical compound formed when the elements sodium and chlorine react together and become joined in an intimate form known as a chemical compound. Air, by contrast, was found to consist of a mixture of the gases nitrogen and oxygen, which did not react with each other.
II DALTON’S THEORY

Water Molecule
A water molecule consists of an oxygen atom and two hydrogen atoms, which are attached at an angle of 105°.
John Dalton, a British schoolmaster and chemist, was fascinated by the patchwork puzzle of the elements. Early in the 19th century he made studies of the way in which the various elements combine with one another to form chemical compounds. Although many other scientists, from the Greeks onward, had already speculated that the smallest units of a substance are atoms, Dalton is regarded as one of the most significant figures in atomic theory because he made the subject quantitative. He showed how these atoms link together in definite proportions. Subsequent investigations proved that atoms normally form groups called molecules. Each molecule of water, for example, consists of a single atom of oxygen and two atoms of hydrogen joined by an electrical force called a chemical bond. Water is symbolized as HOH, or as H2O, meaning that its molecule consists of two atoms of hydrogen joined to one atom of oxygen. See Chemical Reaction.
All atoms of any given element behave in the same way chemically. Thus, from a chemical viewpoint, the atom is the smallest entity to be considered. The chemical properties of the various elements are quite different; their atoms combine in many different ways to form a multitude of different chemical compounds. Some elements, such as the noble gases helium and argon, are inert—that is, they fail to react with other elements except under special conditions. Unlike oxygen, which has a diatomic molecule (two atoms combined in a single molecule), helium and other inert gases are monatomic elements, with a single atom per molecule.
III AVOGADRO’S LAW
The study of gases attracted the attention of the Italian physicist Amedeo Avogadro, who in 1811 formulated an important law bearing his name. This law states that equal volumes of different gases contain the same number of molecules when compared under the same conditions of temperature and pressure. Given these conditions, two identical bottles, one filled with oxygen and the other with helium, will contain exactly the same number of molecules. Twice as many atoms of oxygen will be present, however, because oxygen is diatomic. See also Avogadro’s Law.
IV RELATIVE ATOMIC MASS
It follows from Avogadro’s law that the masses of standard volumes (that is, the densities) of different gases are proportional to the masses of the individual gas molecules. When carbon is taken as a standard and the carbon atom is assigned a value of 12.0000 atomic mass units (symbol u, formerly amu), the hydrogen atom is found to have a mass of 1.0079 u, helium 4.0026 u, fluorine 18.9984 u, and sodium 22.9898 u. Chemists sometimes quote these numbers, without mentioning any unit. These numbers were formerly called “atomic weights”, but are now called “relative atomic masses” (r.a.m.). The new term is preferred because mass is a measure of the quantity of matter in a body, which is the concept that is relevant here; weight is quite different: the force exerted on the body by the influence of gravity.
The observation that many relative atomic masses are close to whole numbers led the British chemist William Prout to suggest in 1816 that all elements might be composed of hydrogen atoms. However, subsequent measurements of relative atomic masses revealed that chlorine, for example, has a r.a.m. of 35.453 u (when carbon is taken as 12). The discovery of such fractional r.a.m. appeared to invalidate Prout’s hypothesis until a century later, when it was discovered that generally the atoms of a given element do not all have the same mass. Atoms of the same element that differ in mass are known as isotopes. In the case of chlorine two isotopes occur in nature. The atoms of one isotope (chlorine-35) have a r.a.m. close to 35 u, while those of the other (chlorine-37) have a r.a.m. close to 37 u. Experiments show that chlorine is a mixture of approximately three parts of chlorine-35 for every one part of chlorine-37. The average r.a.m. of naturally occurring chlorine is therefore approximately (3 × 35 + 37)/4 = 35.5.
During the first part of the 20th century natural oxygen was used as the standard against which atomic masses were measured; oxygen was assigned a r.a.m. of exactly 16. In the early 1960s, the international unions of chemistry and physics agreed on a new standard, assigning a r.a.m. of precisely 12 to the most commonly found isotope of carbon, carbon-12. The new standard is particularly appropriate because carbon-12 is often used as a reference standard when atomic masses are measured with mass spectrometers. The table of relative atomic masses based on carbon-12 is in close agreement with the old table based on natural oxygen.
V THE PERIODIC TABLE

By the middle of the 19th century several chemists had recognized that similarities in the chemical properties of various elements implied a regularity that might be illustrated by arranging the elements in a tabular or periodic form. The Russian chemist Dmitry Mendeleyev proposed a chart of elements called the periodic table (see Periodic Law), in which the elements are arranged in rows and columns so that elements with similar chemical properties are grouped together. According to this arrangement, each element is assigned a number (atomic number) according to its position in the table, ranging from 1 for hydrogen to 92 for uranium, which has the most massive atom of all elements that occur naturally on Earth. Mendeleyev showed enough faith in his table to leave blank spaces where there were no obvious elements to fill the gaps, predicting that new elements would one day be found to go in these places. Further research, aided by the arrangement of the known elements in the chart, led to the discovery of missing elements that fitted the gaps. Elements of higher atomic number have correspondingly heavier r.a.m., and the r.a.m. of each isotope is close to a whole number, in accordance with Prout’s hypothesis.
VI THE SIZE OF THE ATOM

Atoms Made Visible
Atoms are so tiny that they can’t be seen with the naked eye. However, they can be viewed and photographed with a special microscope. This picture shows atoms of the element silicon.
Curiosity about the sizes and masses of atoms tantalized scientists for a long period. Lack of adequate instruments and proper experimental techniques prevented them from obtaining satisfactory answers. In modern times a variety of ingenious techniques have been devised to determine the sizes and masses of the various atoms. The lightest of all atoms, hydrogen, has a diameter of approximately 10-8 cm and a mass of about 1.7 × 10-24 g. An atom is so small that a single drop of water contains more than a thousand billion billion atoms.
VII RADIOACTIVITY
That atoms are not solid bits of matter, incapable of further subdivision, became clear from several major discoveries made near the end of the 19th century. In 1897 the British physicist J. J. Thomson discovered the electron, a particle with much less mass than any atom. Electrons could be produced from a wide variety of elements, and further experimental work gradually showed that it was a building-block of all atoms. In 1896 the French physicist Antoine Henri Becquerel found that certain substances, such as uranium salts, give off penetrating rays of mysterious origin. The French scientists Marie Curie and her husband Pierre Curie contributed further to an understanding of these “radioactive” substances (see Radium). As a result of the research of the British physicist Ernest Rutherford and his contemporaries, it was shown that uranium and some other heavy elements, such as thorium and radium, emit three different kinds of radiation, called alpha (α), beta (β), and gamma (g) rays. The first two types were found to consist of electrically charged bits of matter, now called alpha and beta particles. Alpha particles were later found to be identical to the nuclei, or central parts, of helium atoms (see below), and beta particles were found to be electrons. Gamma rays were eventually identified as electromagnetic waves, similar to X-rays but of shorter wavelengths (see Electromagnetic Radiation).
VIII THE RUTHERFORD NUCLEAR ATOM
Recognition of the nature of radioactive emissions enabled physicists to penetrate more deeply into the atom, which was found to consist mostly of space. At the centre of this space is a core called the nucleus, measuring only about a ten-thousandth of the diameter of the atom. Rutherford established that the mass of the atom is concentrated in its nucleus. He also proposed that the electrons, already known to form part of the atom, travel in orbits around the nucleus. The nucleus has a positive electrical charge; the electrons each have a negative charge. The charges carried by the electrons add up to the same amount of electricity as those residing in the nucleus, and thus the normal electrical state of the atom is neutral. See Physics: Atomic Models.
IX THE ELECTRON STRUCTURE OF THE ATOM

Electron Density and Orbital Shapes
Atomic orbitals are mathematical descriptions of where the electrons in an atom (or molecule) are most likely to be found. These descriptions are obtained by solving an equation known as the Schrödinger equation, which expresses our knowledge of the atomic world. The orbitals shown illustrate the spatial distribution of electrons with increasing (s,p,d,f) amounts of angular momentum. The fundamental nature of electrons prevents more than two from ever being in the same orbital. The overall distribution of electrons in an atom is the sum of many such pictures. This description has been confirmed by many experiments in chemistry and physics, including an actual picture of a p-orbital made by a scanning tunneling microscope.
To explain the structure of the atom, the Danish physicist Niels Bohr developed in 1913 a hypothesis known as the Bohr theory of the atom (see Quantum Theory). He assumed that electrons move in definite orbits at a considerable distance from the nucleus. The number of such electrons equals the atomic number of the atom: hydrogen has a single orbital electron, helium has 2, and uranium has 92. Bohr was able to use his model of the atom to explain the spectrum of the simplest atom, hydrogen (see below).
Bohr’s ideas were developed further to explain the chemical properties of the elements. It was realized that the electrons’ orbits are grouped together into “shells”, each of which has an upper limit to the number of electrons that it can accommodate. The first shell is complete when it contains two electrons, the second can hold up to eight, and successive shells hold still larger numbers. The seventh shell is not filled in any naturally occurring atom. The “last” electrons, those which are outermost or added last to the atom’s structure, determine the chemical behaviour of the atom.

Models of the Atom
Experimental data have been the impetus behind the creation and dismissal of physical models of the atom. Rutherford’s model, in which electrons move around a tightly packed, positively charged nucleus, successfully explained the results of scattering experiments, but was unable to explain discrete atomic emission—that is, why atoms emit only certain wavelengths of light. Bohr began with Rutherford’s model, but then postulated further that electrons can move only in certain quantized orbits; this model was able to explain certain qualities of discrete emission for hydrogen, but failed for other elements. Schrödinger’s model, in which an electron is described not in terms of definite paths but in terms of the likelihood of finding the electron in a particular region, can explain certain qualities of emission spectra for all elements; however, further refinements of the model, made throughout the 20th century, have been needed to explain further spectral phenomena.
The inert, or noble, gases (helium, neon, argon, krypton, xenon, and radon) all have completely filled outer shells. They do not enter into chemical combinations in nature, although the three heaviest inert gases (krypton, xenon, and radon) have formed chemical compounds in the laboratory. On the other hand, the outermost shells of such elements as lithium, sodium, and potassium contain only one electron. These elements combine readily with other elements (transferring their outermost electrons to them) to form a great many chemical compounds. Correspondingly, such elements as fluorine, chlorine, and bromine lack only one electron to acquire a filled outermost shell. They too combine readily with other elements, receiving electrons from them.
Atomic shells do not necessarily fill up with electrons in consecutive order. The electrons of the first 18 elements in the periodic table are added in a regular manner, each shell being filled to a designated limit before a new shell is started. From the 19th element onwards, the outermost shell is started before the previous shell is completely filled. A regularity is still maintained, however, as electrons fill successive shells in a repetitive back-and-forth pattern. The result is the regular repetition of chemical properties for atoms of increasing atomic weight that corresponds to the arrangement of the elements in the periodic table.
It is still convenient to visualize the electrons as Rutherford and Bohr did originally, as moving about the nucleus of an atom much as if they were planets moving about the Sun. This view is much simpler than that held by present-day physicists, however. It is now known that it is impossible to pinpoint the precise position of an electron in the atom’s space without disturbing its predicted location at some future time. This uncertainty is expressed by attributing to the atom a cloud-like form, in which the electron’s position is defined in terms of the probability of finding it at some distance from the nucleus. The “probability-cloud” view of the atom has superseded the solar-system model.
X LINE SPECTRA
One of the great successes of theoretical physics was the explanation of the characteristic line spectra of various elements (see Spectroscopy: Spectrum Lines). Atoms excited by a supply of energy from an external source emit light of well-defined frequencies. If hydrogen gas, for example, is held at low pressure in a glass tube and an electrical current is passed through it, visible light of a reddish colour is given off. Careful examination of this light with a spectroscope shows a line spectrum, a series of regularly spaced lines of light. Each line is an image of the spectroscope’s slit, formed in a particular colour of light. Each line has a definite wavelength and associated energy. For the simplest kind of atom, the hydrogen atom, the Bohr theory permits the physicist to calculate these wavelengths in a straightforward fashion. It is assumed that in the atom an electron can move only in a “permitted” orbit. Although there are infinitely many of these, they are discrete (separate)—intermediate orbits are not possible. While an electron remains in an orbit at a fixed distance from the nucleus, the atom does not radiate energy. When the atom is excited, the electron jumps to a higher-energy orbit farther from the nucleus. When it falls back to an orbit closer to the nucleus, it emits a discrete amount of energy corresponding to a certain wavelength of light. The electron may return to its original orbit in several steps, via orbits that are not fully occupied. Each line observed represents an electronic transition between orbits of higher and lower energy. For atoms more complex than that of hydrogen, the simple Bohr theory fails. However, the spectra of such atoms are successfully explained by the more sophisticated quantum theory that developed later.
If the atoms of an element of high atomic number are so highly excited that inner electrons close to the nucleus are affected, then penetrating radiation—X-rays—will be emitted. These electronic transitions involve large amounts of energy.
XI THE ATOMIC NUCLEUS
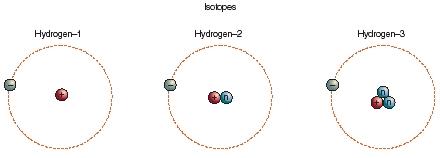
Hydrogen Isotopes
The atomic number of an atom represents the number of protons in its nucleus. This number remains constant for a given element. The number of neutrons, however, may vary, creating isotopes that have the same chemical behaviour, but different mass. Hydrogen always has one proton in its nucleus, which is balanced by one electron. The isotopes of hydrogen are protium (no additional neutrons), deuterium (one neutron), and tritium (two neutrons). These pictures are schematic representations of the atom. In reality the nucleus is 10,000 times smaller than the atom, and the electron is a million times smaller than the nucleus. The size of the atom is determined by the motion of the electron, which occurs in regions of space called orbitals.
In 1919 Rutherford exposed nitrogen gas to a radioactive source that emitted alpha particles. Some of the alpha particles collided with the nuclei of the nitrogen atoms. As a result of these collisions, the nitrogen atoms were transmuted into oxygen atoms. A positively charged particle was emitted from the nucleus of each of the atoms undergoing transmutation. These particles were recognized as being identical to the nuclei of hydrogen atoms. They are called protons. Further research proved that protons are constituents of the nuclei of all elements.
No more clues to the structure of the nucleus were found until 1932, when the British physicist Sir James Chadwick discovered another particle, known as the neutron, having almost exactly the same weight as the proton but without an electrical charge. The neutron was emitted from certain atoms in nuclear reactions (see below). It was realized that the nucleus is made up of protons and neutrons. In any given atom, the number of protons is equal to the number of electrons and hence to the atomic number of the atom. Isotopes are then explained as atoms of the same element (that is, containing the same number of protons) that have different numbers of neutrons. In the case of chlorine, one isotope is identified by the symbol 35Cl and its heavy relative by 37Cl. The superscripts identify the mass number of the isotope and are numerically equal to the total number of neutrons and protons in the nucleus of the atom. Sometimes the atomic number is given as a subscript, as in ·Cl.
The lighter stable isotopes (apart from hydrogen, whose main isotope contains a single proton and no neutrons) have approximately equal numbers of protons and neutrons, but for stable isotopes of heavier elements more neutrons are required to counteract the repulsive force between protons which would disrupt the nucleus. The heaviest stable isotopes have about 1.5 times as many neutrons as protons.
XII ARTIFICIAL RADIOACTIVITY
In addition to the naturally occurring radioactive isotopes, stable atoms of an element may be made artificially radioactive by suitable bombardment with nuclear particles or rays. Such radioactive isotopes (radioisotopes) are produced as a result of a nuclear reaction, or transformation. In such reactions the 270-odd isotopes found in nature serve as targets for nuclear projectiles. The development of accelerators, for hurling these projectile-particles to high energy, has made it possible to observe thousands of nuclear reactions.
XIII NUCLEAR REACTIONS
In 1932 two British scientists, Sir John D. Cockcroft and Ernest T. S. Walton, were the first to use artificially accelerated particles to disintegrate the nucleus. They produced a beam of protons, which were boosted to high speed by means of a high-voltage device called a voltage multiplier. These particles were then used to bombard a lithium target. In this nuclear reaction, lithium-7 (7Li) splits into two fragments, which are nuclei of helium atoms. The reaction is expressed by the equation 7Li + 1H = 4He + 4He
Since then thousands of nuclear reactions have been studied. The sums of the masses of the particles on the two sides of such equations are not precisely equal. If the reaction products on the right-hand side have a smaller sum, then the difference is made up by the kinetic energy of the reaction products, according to the Einstein relation E = mc2 (see below). If this sum is greater, then appropriate kinetic energy must be supplied by the bombarding particle. See Nuclear Physics.
XIV PARTICLE ACCELERATORS
In about 1930 the American physicist Ernest O. Lawrence developed a particle accelerator called a cyclotron. This machine generates attractive and repulsive electrical forces that accelerate nuclei (usually protons) while they are confined to circular orbits by the field of a large electromagnet. The particles spiral outward under the influence of these forces, reaching energies of up to several hundred MeV (million electronvolts, or 106 eV). The acceleration takes place in a vacuum so that the particles do not collide with molecules of air, which would result in losses. Over the years, other types of particle accelerators of increasing energy have been developed, the largest of which accelerate particles to the TeV (1012 eV) range. These largest machines have dimensions of several kilometres, are very complex and expensive, are used by large international collaborations, and are sometimes constructed and funded internationally.
XV ELEMENTARY PARTICLE PHYSICS

Elementary Particle Tracks
These tracks were formed by elementary particles in a bubble chamber at the CERN facility on the Swiss-French border near Geneva. By examining these tracks, physicists can determine certain properties of particles that travelled through the bubble chamber. For example, a particle’s charge can be determined by noting the type of path the particle followed. The bubble chamber is placed within a magnetic field, which causes a positively charged particle’s track to curve in one direction, and a negatively charged particle’s track to curve the opposite way; neutral particles, unaffected by the magnetic field, move in a straight line.
When high-energy particles hit nuclei, new unstable particles can be created. These were first observed in interactions of cosmic rays with nuclei. These rays consist of highly energetic particles that constantly bombard the Earth from outer space. With the advent of particle accelerators starting in the 1950s, more and more such new particles were found. When the number of different particles reached 200, greatly exceeding the number of chemical elements, it no longer seemed appropriate to use the term “elementary”, and indeed a new layer of “elementarity” was discovered.
Elementary particle physics has two main aims: to understand the ultimate building blocks of matter, and to understand the forces through which these interact. Interestingly, there are now close links with astrophysics and cosmology, since very high-energy collisions were commonplace in the very early universe. There are four fundamental forces: the strong force, electromagnetism, the weak force, and gravity.
There appear to be only two types of building block: quarks and leptons. Quarks, which were first proposed independently by American physicists Murray Gell-Mann and George Zweig, feel the strong force, while leptons do not. Normal matter only requires two types of quark called “up” and “down” having electric charges +’e and –€e, where –e is the charge of the electron. Protons consist of two up and one down quark, while neutrons consist of one up and two down quarks. The electron is a lepton, and so is the neutrino (a neutral particle found in some radioactive decays). In high-energy collisions, more quarks and leptons are found, making a total of six of each. All quarks and leptons have antiparticles (see Antimatter). The quarks and the charged leptons feel the electromagnetic force. All elementary particles feel the weak force and gravity.
According to quantum theory, the forces are “carried” (mediated is the technical term) by particle-like objects called bosons. Electromagnetism is carried by photons, while gravity is believed to be carried by gravitons, although these have not yet been discovered. The strong force is carried by gluons and the weak force is carried by W and Z bosons. Theories proposed independently by Abdus Salam in the United Kingdom and Steven Weinberg and Sheldon Glashow in the United States suggested that electromagnetism and the weak force were closely related, that is, at high energies they were effectively the same. All three shared the 1979 Nobel Prize for Physics for their work. The discovery of the W and Z bosons at the European Laboratory for Particle Physics (CERN) in 1983 was the experimental verification of electroweak unification. Italian Carlo Rubbia and Dutchman Simon van der Meer, both of CERN, shared the 1984 Nobel Prize for Physics for their part in this discovery.
XVI THE RELEASE OF ATOMIC ENERGY
In 1905 Albert Einstein developed his mass-energy equation, E = mc2, as part of his special theory of relativity. This equation states that with a given mass (m) is associated an amount of energy (E) equal to this mass multiplied by the square of the velocity of light (c). A very small amount of mass is equivalent to a vast amount of energy. Because more than 99 per cent of the atom’s mass is in the nucleus, any release of large amounts of the atom’s energy has to come from the nucleus.
Two nuclear processes of great practical significance because they provide vast amounts of energy are fission, the splitting of a heavy nucleus into lighter ones, and thermonuclear fusion, the fusion of two light nuclei (at extremely high temperatures) to form a heavier one. The Italian-born American physicist Enrico Fermi achieved fission in 1934, but the reaction was not recognized as such until 1939, when the German scientists Otto Hahn and Fritz Strassmann announced that they had split uranium nuclei by bombarding them with neutrons. Neutrons are also released by the reaction and can cause a chain reaction with other nuclei. An uncontrolled chain reaction is seen in the explosion of an atomic bomb. Heat from controlled reactions, however, as in nuclear reactors, can be used to produce electric power.
Thermonuclear fusion occurs in stars, including the Sun, and is the source of their heat and light. Uncontrolled fusion is seen in the explosion of a hydrogen bomb, but physicists are currently trying to develop a practical controlled-fusion device. See Nuclear Energy; Nuclear Weapons.